Principal Investigator

Staff
Menglì Yue
Research topics
Pancreatic cancer is among the deadliest malignancies, but unlocking its hidden diversity and reprogramming its hostile microenvironment could finally turn the tide toward effective immunotherapy.
Background
More than half a million new cases of pancreatic ductal adenocarcinoma (PDAC), the most prevalent and aggressive form of pancreatic cancer, are diagnosed each year. This makes PDAC the third leading cause of cancer-related deaths worldwide – and it is projected to rise to second place by 2030.
Despite major advances in precision medicine and immunotherapy for other cancers, PDAC has seen limited progress. PDAC is difficult to treat: it displays few recognizable antigens, is encased in dense fibrotic tissue, and creates a profoundly immunosuppressive microenvironment that prevents immune cells – including engineered CAR-T cells – from entering and acting effectively.
Adding to this challenge, PDAC tumours are not uniform. They evolve as mosaics of subclones, including small stem-like populations that adapt and resist treatment. These hidden subpopulations often escape conventional analyses and undermine current therapies. Addressing this cellular diversity is critical to achieving durable treatment responses.
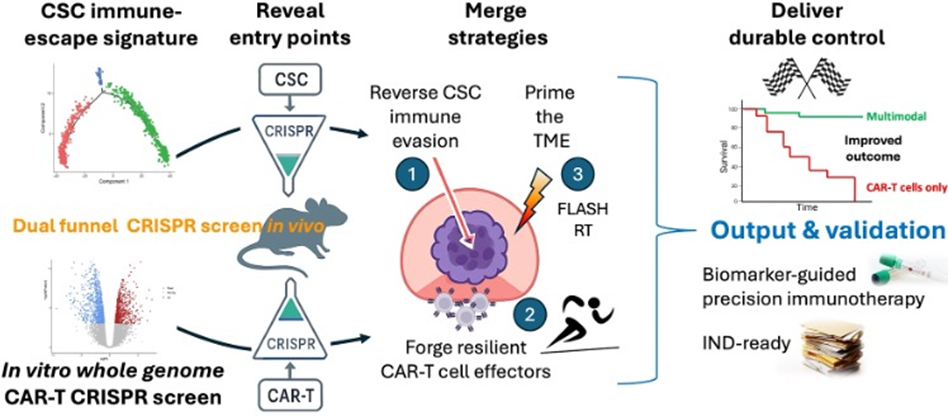
To overcome these barriers, new approaches must revert stemness and immune evasive properties of PDAC cells, increase tumour antigen visibility, and reprogram the tumour microenvironment. The aim is to transform immunologically “cold” PDAC into “hot” tumours that can be recognized and attacked by the (engineered) immune system.
Research achievements
- Dissecting Non-Genetic Evolution in Pancreatic Cancer with Single-Cell Omics
We have shown for the first time that the molecular and functional heterogeneity of PDAC is driven in part by a distinct subset of cells with enhanced stem-like properties. Our research has shown that these cancer stem cells (CSCs) are uniquely capable of sustaining tumour growth, much like normal stem cells fuel the development of healthy tissue. Remarkably, even a single CSC can regenerate a heterogeneous PDAC tumour, maintain its tumorigenic potential through serial transplantation, and play a central role in metastasis and drug resistance.
Subsequently, we have demonstrated that disrupting stemness and immune evasion dramatically improves responses to standard therapies. This highlights a promising strategy: by targeting the transcriptional circuits that sustain stemness, it may be possible to prevent relapse and achieve durable treatment outcomes in pancreatic cancer.
- Developing Novel CAR T-Cell Immunotherapies
Our laboratory is now advancing precision immunotherapies by expanding the repertoire of CAR T-cells directed against both universal and cancer-specific surface antigens.
One of our newly identified targets plays a critical role in cellular proliferation and tumour migration yet shows minimal expression in normal tissues – making it a highly versatile candidate for safe and effective therapy. Another promising antigen, previously recognized in cancer biology, revealed a unique localization pattern in our studies that may establish it as a broadly applicable cancer-specific target. We are currently evaluating the therapeutic potential of these novel CAR T-cells in vivo across diverse models of metastatic disease.
Conclusion and perspectives
Large-Scale and High-Resolution Interrogation of Cancer Stem Cells
At the Candiolo Cancer Institute, we are leveraging fresh patient material to explore how pancreatic cancer evolves and resists therapy through non-genetic mechanisms. By combining cutting-edge single-cell technologies developed in our laboratory with advanced bioinformatics, we aim to uncover the regulatory networks that drive stemness, metabolism, and immune evasion. Our goal is to translate these insights into novel therapeutic strategies that improve long-term outcomes for patients.
Specific aims of the project:
- Dissect the heterogeneity of PDAC by applying high-resolution single-cell omics to primary tumours and circulating tumour cells (CTCs);
- Prospectively identify CSCs and validate novel therapeutic targets using advanced bioinformatic and molecular approaches;
- Develop multimodal strategies to overcome stemness and immune evasion;
- Validate new treatment approaches through CTC-guided precision medicine.
Developing Advanced CAR-T Cell Immunotherapies for Pancreatic Cancer
This research program is built on the hypothesis that reprogramming the immune evasion mechanisms of pancreatic cancer will unlock the full potential of novel CAR-T cell therapies. By integrating cutting-edge immunology, molecular biology, and nanotechnology, we aim to design next-generation precision immunotherapies.
Specific aims of the project:
- Develop and validate 4th-generation CAR-T cells engineered to resist exhaustion and efficiently target novel cancer-specific antigens;
- Characterize and modulate the immune landscape of pancreatic cancer using advanced multiplex technologies;
- Reprogram the tumour microenvironment through targeted treatments and nanoparticle-based approaches;
- Combine immune reprogramming strategies with novel CAR-T cells to enhance therapeutic efficacy.
Our ultimate goal is to create multimodal treatment regimens capable of rapidly slowing disease progression and potentially doubling (or more) the median survival of PDAC patients. The precision immunotherapy platforms developed in this program may also provide new therapeutic avenues for other cancers with urgent unmet needs.
Publications
At this link, you can find all the scientific publications of the Principal Investigator.
Selected publications
Patrick C Hermann 1, Stephan L Huber, Tanja Herrler, Alexandra Aicher, Joachim W Ellwart, Markus Guba, Christiane J Bruns, Christopher Heeschen
Irene Miranda-Lorenzo, Jorge Dorado, Enza Lonardo, Sonia Alcala, Alicia G Serrano, Jenifer Clausell-Tormos, Michele Cioffi, Diego Megias, Sladjana Zagorac, Anamaria Balic, Manuel Hidalgo 4, Mert Erkan, Joerg Kleeff 5, Aldo Scarpa, Bruno Sainz Jr, Christopher Heeschen
FNDC4 Drives Metastasis and Immune Evasion in Pancreatic Cancer
Li J, Wu R, Jin X, Heeschen
Qing Y., Jiang K., Jiang H., Heeschen C.
Raj D, Yang MH, Rodgers D, Heeschen C